Abstract
Background: Priapism is a painful, sustained penile erection that occurs in 35% of adult males with sickle cell disease (SCD); priapism may result from the underlying mechanism of microcapillary vaso-occlusion. To date, there is no effective SCD modifying drug proven to treat priapism. Crizanlizumab is a monoclonal antibody that binds and blocks P-selectin; a key mechanistic component of the vaso-occlusive process. Based on the results of the SUSTAIN trial, crizanlizumab was approved by the US Food and Drug Administration to reduce the frequency of vaso-occlusive crises in SCD patients aged 16 years and older. In this interim analysis of the SPARTAN trial (NCT03938454), we evaluate the efficacy and safety of crizanlizumab in SCD patients with priapism.
Methods: The SPARTAN trial included patients aged ≥12 years with all SCD genotypes with ≥4 priapic episodes lasting ≥60 minutes over the 14 weeks preceding the study. Eligibility criteria further required participants to experience ≥3 priapic events during the subsequent 12-week screening period with ≥1 event occurring within 4 weeks prior to the first treatment. Priapic events were documented through electronic self-reporting tools as well as validated patient-reported outcomes surveys for 26 weeks and included start/end date, sleep status, duration, non-pharmacological treatment, triggers, emergency department visit and pain intensity. After enrollment baseline priapic events were collected for each patient during the 12-week screening period. Patients received crizanlizumab 5.0 mg/kg intravenous infusion at week 1, week 3 and every 4 weeks thereafter. The primary endpoint was the percent reduction from baseline in frequency of priapic events by 26 weeks. All analyses were descriptive.
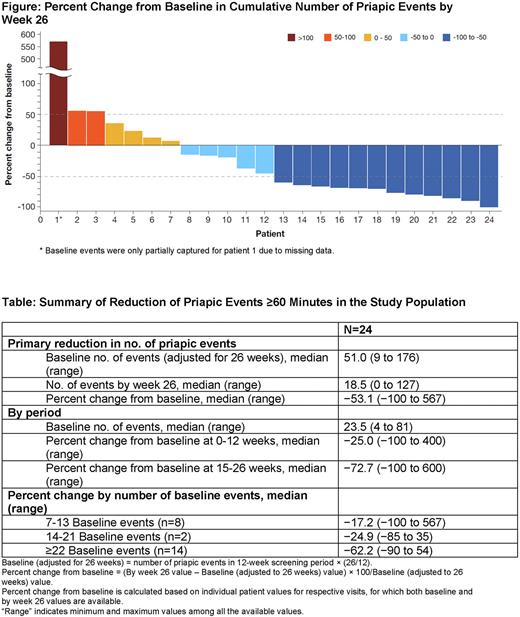
Results: At the interim analysis (data cut off, March 8, 2022) 24 patients had received ≥1 dose of crizanlizumab and 3 patients missed 1 infusion each. Most patients (70.8%) were between the ages of 18 and 40 years, and the median (range) age was 29.5 years (17-58 years). All patients were African American, and most had HbSS genotype (79.2%). More than half the patients (58.3%) reported receiving hydroxyurea therapy prior to enrollment and remained on a stable dose throughout the study period. The first episode of priapism occurred between the ages of 13 and 25 years in 79.2% of the patients. Frequency and length of priapic events varied with no specific pattern; however, nearly all patients (95.8%) reported priapism-associated pain. Common nonpharmacological methods that helped priapism episodes were shower or bath (58.3%) and exercise (58.3%). The most commonly reported patient-reported emotions at the start of the study were exhaustion (50%), frustration (45.8%), embarrassment (41.7%) and anxiousness (41.7%). The median (IQR) baseline number of priapic events adjusted for 26 weeks per patient was 51 events (11-74 events) (Table). By week 26, a reduction in priapic events occurred in 17 of 24 patients (70.8%). The median (IQR) percent reduction from baseline in priapic events per patient was −53.1% (−73.4% to 9.3%). There was a higher median (IQR) percent reduction from baseline during weeks 15 to 26 (−72.7% [−94.0% to −37.1%]) as compared to weeks 0 to 12 (−25.0% [−52.9% to 1.5%]). In a subgroup analysis by frequency of baseline priapic events, the largest benefit was observed in patients with ≥22 baseline events (median [IQR], −62.2% [−76.3% to −17.6%]). Results for each patient are presented in the Figure. Crizanlizumab was well tolerated with the 2 most frequent treatment-emergent adverse events (TEAEs) being headache (16.7%) and fatigue (12.5%). No patients discontinued crizanlizumab prematurely.
Conclusion: Interim data from the phase 2 SPARTAN trial show that patients with SCD-related priapism treated with crizanlizumab over 26 weeks experienced approximately half as many priapic events compared with baseline. There was a trend toward improved efficacy with a longer treatment period and in patients with a higher number of events at baseline. Crizanlizumab was safe to use and well tolerated with observed TEAEs consistent with the safety profile seen in other clinical trials with crizanlizumab. Final results of the SPARTAN trial will provide more definitive conclusions regarding the efficacy of crizanlizumab in decreasing priapism.
Disclosures
Anderson:Global Blood Therapeutics: Consultancy, Speakers Bureau. El Rassi:Novartis, Forma Therapeutics, Agios: Membership on an entity's Board of Directors or advisory committees, Research Funding. Idowu:Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Ironwood: Research Funding; Forma: Research Funding; Pfizer: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees. Kanter:Novartis: Consultancy, Honoraria; GLG: Consultancy; Ecor1: Honoraria; Guidepoint Global: Honoraria; Beam: Honoraria; Graphite Bio: Consultancy; ORIC: Consultancy; Bausch: Consultancy, Honoraria; University of Alabama Birmingham: Current Employment; Forma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Fulcrum Tx: Consultancy. Curtis:Global Blood Therapeutics: Consultancy, Honoraria; Novartis: Honoraria. Liles:Vifor: Other: presently and the Sub I or PI on trials; Biogene: Other: presently and the Sub I or PI on trials; Novartis: Other: presently and the Sub I or PI on trials; Takeda: Other: presently and the Sub I or PI on trials; Shire,: Other: presently and the Sub I or PI on trials; Incye,: Other: presently and the Sub I or PI on trials; Apellis: Other: presently and the Sub I or PI on trials; Sun Pharma: Other: presently and the Sub I or PI on trials; Momenta pharmaceuticals: Other: presently and the Sub I or PI on trials; PiCori: Other: I also am the Site Sub I for 2 PiCori grants in sickle cell anemia.. Andemariam:Novo Nordisk A/S: Consultancy, Membership on an entity's Board of Directors or advisory committees; Hemanext: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Aruvant: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi Genzyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Emmaus: Consultancy, Membership on an entity's Board of Directors or advisory committees; CRISPR Therapeutics AG: Consultancy, Membership on an entity's Board of Directors or advisory committees; Shenox: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Forma Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Vertex: Consultancy, Membership on an entity's Board of Directors or advisory committees; Terumo BCT: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees. Paulose:Novartis Pharmaceuticals Corporation: Current Employment, Current equity holder in publicly-traded company. Laine:Novartis Pharmaceuticals Corporation: Current Employment. Khan:Novartis: Current Employment, Current equity holder in publicly-traded company. Darbari:Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Burnett:Endo Pharmaceuticals: Research Funding; National Institutes of Health: Research Funding; Boston Scientific: Consultancy, Honoraria, Research Funding; Novartis Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Futura Medical: Research Funding; Myriad Genetics: Research Funding; Comphya: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal